Related Articles
00 / 00
-

Dr. Lori Rink in a lab
-

-

Margaret von Mehren, MD
-


This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.




Gastrointestinal Stromal Tumors (GIST) are the most common sarcomas of the gastrointestinal tract. These tumors are characterized by near-universal expression of the receptor tyrosine kinase (RTK) KIT, and the majority of GIST harbor constitutively active mutant isoforms of KIT (70-80%) or the related RTK, PDGFRA (5-7%). The ~10-15% of GIST that lack mutations in these genes often exhibit genetic or epigenetic deficiencies in the succinate dehydrogenase (SDH) complex of the respiratory chain, and are referred to as SDH-deficient (SDH-d). Therapeutic targeting of GIST with the front-line RTK inhibitor, imatinib mesylate (IM) along with four other FDA approved agents (sunitinib, regorafenib, ripretinib and avapritinib) has transformed therapy for advanced, unresectable GIST. Although these RTK inhibitors are effective in most GIST, primary and acquired resistance remains a significant clinical obstacle. Our research is focused on elucidating mechanisms of resistance to these RTK inhibitors and identifying and evaluating new therapeutic targets in preclinical models of GIST.
Gastrointestinal Stromal Tumors (GIST) are the most common sarcomas of the gastrointestinal tract. These tumors are characterized by near-universal expression of the receptor tyrosine kinase (RTK) KIT, and the majority of GIST harbor constitutively active mutant isoforms of KIT (70-80%) or the related RTK, PDGFRA (5-7%). The ~10-15% of GIST that lack mutations in these genes often exhibit genetic or epigenetic deficiencies in the succinate dehydrogenase (SDH) complex of the respiratory chain, and are referred to as SDH-deficient (SDH-d). Therapeutic targeting of GIST with the front-line RTK inhibitor, imatinib mesylate (IM) along with four other FDA approved agents (sunitinib, regorafenib, ripretinib and avapritinib) has transformed therapy for advanced, unresectable GIST. Although these RTK inhibitors are effective in most GIST, primary and acquired resistance remains a significant clinical obstacle. Our research is focused on elucidating mechanisms of resistance to these RTK inhibitors and identifying and evaluating new therapeutic targets in preclinical models of GIST.
A role for AKT in drug resistance in GIST
Following a period of response to RTK therapy, the majority of tumors ultimately develop resistance, mainly through the acquisition of secondary RTK mutations and the activation of bypass RTK downstream pathways, such as the PI3K/AKT pathway. Unfortunately, once all approved lines of therapy have failed, patients with advanced GIST are then left without any further treatment options. Therefore, a significant problem in the treatment of GIST is preventing the development of acquired resistance to the most potent first-line therapeutic agent, IM.
We demonstrated AKT activation to be a hallmark of IM resistance in GIST. The presence of an additional therapeutic agent could potentially suppress the growth and survival of a clone of cancer cells, which have developed resistance to IM. Simultaneous targeting of AKT in addition to inhibiting KIT with IM has potential to be a promising strategy to prevent the development of secondary resistance to IM. Our group has previously shown significantly enhanced combination effects of IM with MK-2206, an oral pan-AKT inhibitor, in an IM-sensitive xenograft model in vivo, as measured by extended disease stabilization and improved survival. We are currently evaluating the efficacy of several new AKT inhibitors in both IM-sensitive and resistant preclinical GIST models and interrogating the mechanism of synergy resulting from this drug combination.
Kinome profiling in GIST to identify new targets
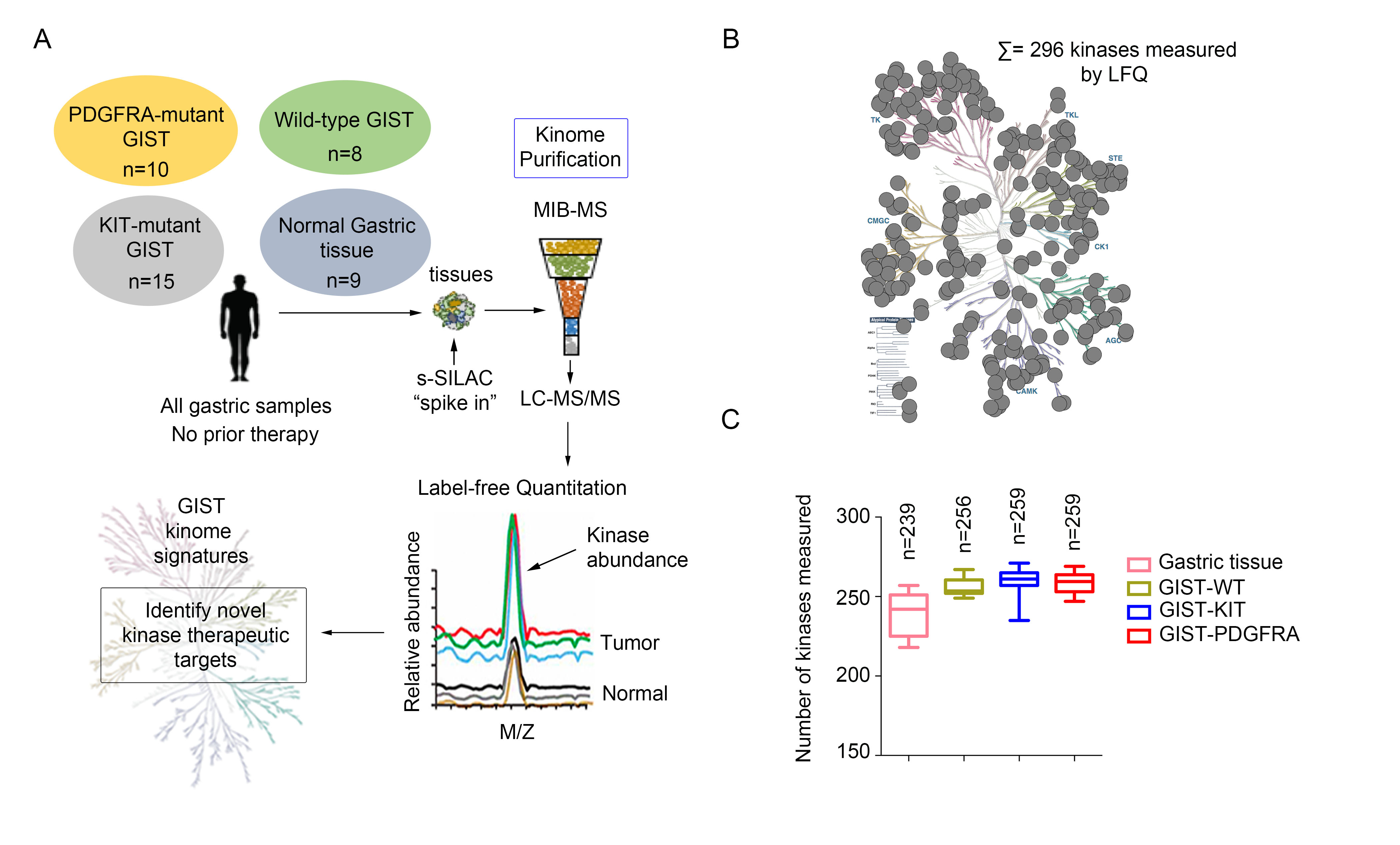
GIST has three major genotypic subtypes: KIT mutant, PDGFRA mutant and SDH-deficient GIST. Mutational subsets confer different natural histories and primary response to IM. Limited options exist for those patients whose disease is refractory to primary treatment. Therefore, there is an urgent need to identify novel targets to provide additional therapeutic options and combinations for refractory GIST. Inhibition of cancer-promoting cell signaling pathways by targeting additional protein kinases active in GIST may provide these options. Global kinome profiling has the potential to identify critical signaling networks and reveal protein kinases that are essential in GIST. In collaboration with Dr. James Duncan (Cancer Biology Program, Fox Chase Cancer Center), we explored the majority of the kinome in GIST specimens from the three most common molecular subtypes to identify novel kinase targets using multiplexed inhibitor beads and mass spectrometry. Using this proteomics approach, we demonstrated that the three GIST subtypes have distinct kinome profiles, and identified kinases that are universally activated in all GIST, as well as kinases that are unique to each subtype. We are currently working to evaluate the identified hits as potential novel targets in GIST.
Identification of Wee1 as a target in combination with avapritinib for GIST treatment
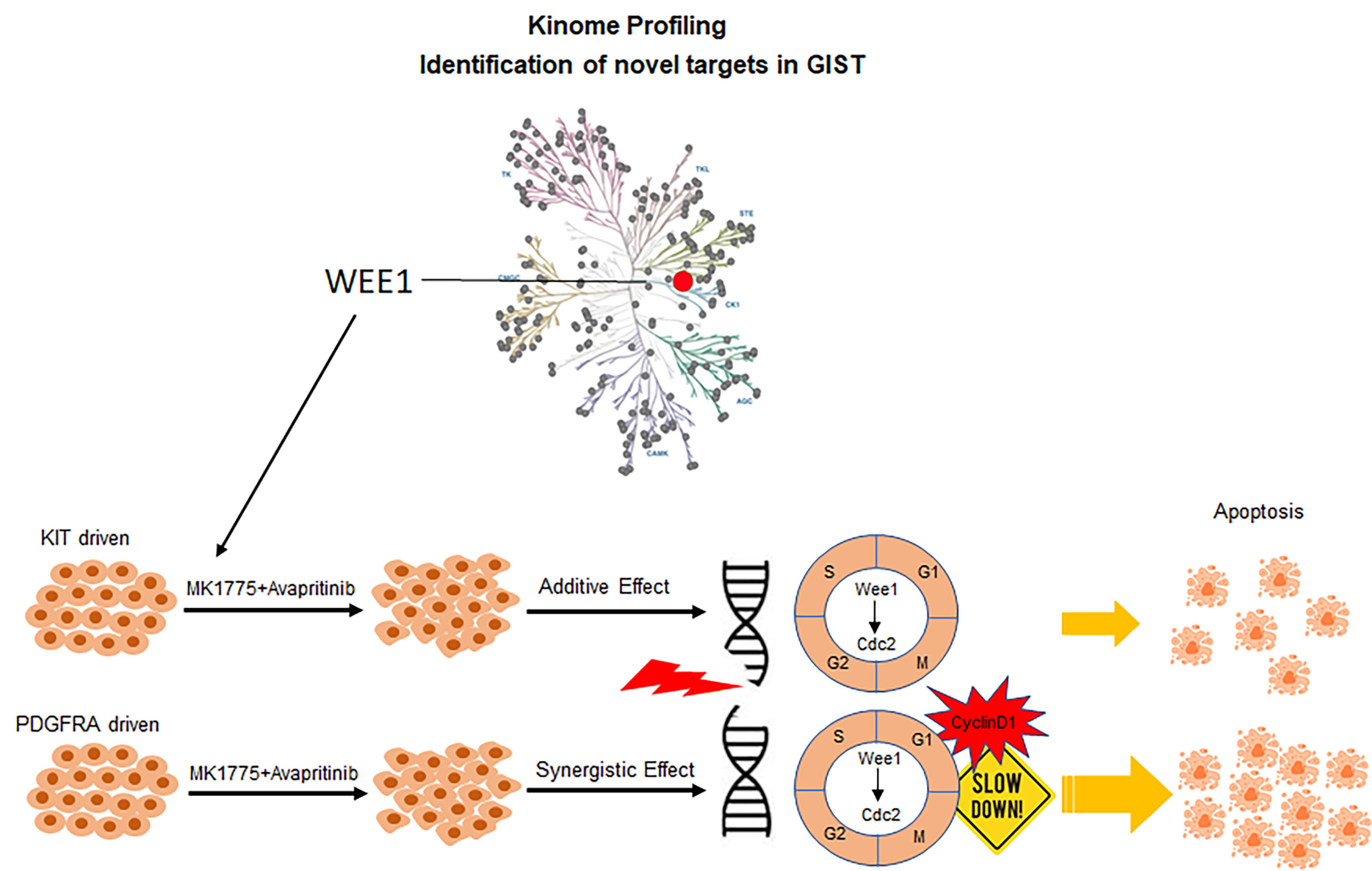
Wee1, gatekeeper of the G2/M cell cycle checkpoint, was shown to be highly abundant in GIST compared to normal tissue as part of our kinome profiling study. Loss-of-function studies targeting Wee1 in KIT and PDGFRA-mutant GIST cell lines revealed an essential role for Wee1 in GIST cell proliferation, suggesting Wee1 as a plausible drug target in GIST. We demonstrated enhanced drug combination effects between avapritinib and MK-1775 (Wee1 inhibitor) in both KIT and PDGFRA-driven GIST cell lines using two-dimensional (2D) and three-dimensional (3D) in vitro viability studies. Mechanistic studies revealed that this drug combination led to increased DNA damage and a loss of cell cycle regulation, decreasing time for DNA damage repair, and ultimately causing increased apoptosis. In vivo studies demonstrated that dual inhibition of Wee1 and KIT/PDGFRA in GIST xenografts provided impressive, extended disease stabilization and improved survival.
Development of novel patient-derived xenograft (PDX) models of GIST.
A limitation to the study of GIST is the lack of available models to study in the laboratory. There are limited cell lines that have been developed, and attempts at developing them have been very challenging. One method to overcome this difficulty has been to implant tumor tissue removed at the time of surgery and directly implant the tissue into mice. This approach, called PDX models, is one that we have been taking in collaboration with our surgical oncology colleagues. To date we have successfully developed eight models. Once developed, this approach allows for testing of novel drugs and drug combinations in animal models representing different genotypes to expand the available models for further preclinical testing of novel agents and or combinations of agents.
Kozinova M, Joshi S, Ye S, Belinsky MG, Sharipova D, Farma JM, Reddy SS, Litwin S, Devarajan K, Campos AR, Yu Y, Schwartz B, von Mehren M, Rink L. Combined Inhibition of AKT and KIT Restores Expression of Programmed Cell Death 4 (PDCD4) in Gastrointestinal Stromal Tumor. Cancers. 2021 Jul 23;13(15). doi: 10.3390/cancers13153699. PubMed PMID: 34359600; PubMed Central PMCID: PMC8345102.
Ye S, Sharipova D, Kozinova M, Klug L, D’Souza J, Belinsky MG, Johnson KJ, Einarson MB, Devarajan K, Zhou Y, Litwin S, Heinrich MC, DeMatteo R, von Mehren M, Duncan JS, Rink L. Identification of Wee1 as Target in Combination with Avapritinib for Gastrointestinal Stromal Tumor. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.143474.
von Mehren M, George S, Heinrich MC, Schuetze SM, Yap JT, Yu JQ, Abbott A, Litwin S, Crowley J, Belinsky M, Janeway KA, Hornick JL, Flieder DB, Chugh R, Rink L, Van den Abbeele AD. Linsitinib (OSI-906) for the Treatment of Adult and Pediatric Wild-Type Gastrointestinal Stromal Tumos, a SARC Phase II Study. Clinical Cancer Research. 26(8):1837-1845, 2020. https://pubmed.ncbi.nlm.nih.gov/31792037/
Zook P, Pathak HB, Belinsky MG, Gersz L, Devarajan K, Zhou Y, Godwin AK, von Mehren M, Rink L. Combination of Imatinib Mesylate and AKT inhibitor provides synergistic effects in preclinical study of Gastrointestinal Stromal Tumor. Clinical Cancer Research. 23(1);171-180, 2017. PubMed
Hensley H., Devarajan K., Johnson J.R., Piwnica-Worms D., Godwin A.K., von Mehren M., Rink L. Evaluating new therapies in Gastrointestinal Stromal Tumor using in vivo molecular optical imaging. Cancer Biol. & Therapy. Jul; 15(7):911-8, 2014. PubMed
Rink L., Ochs M.F., Zhou Y., von Mehren M., Godwin A.K. ZNF-mediated resistance to imatinib mesylate in gastrointestinal stromal tumor. PLoS One. 8(1):e54477, 2013. PubMed
Rink, L., Skorobogatko, Y., Kossenkov, A., Belinksy, M., Pajak, T., Heinrich, M.C., Ochs, M., von Mehren, M., Eisenberg, B., and Godwin, A.K. Gene expression signatures and response to Imatinib Mesylate in Gastrointestinal Stromal Tumors. Mol. Cancer Ther. 8:2172-2182, 2009. PubMed
Tarn, C.┼, Rink, L. ┼, Merkel, E., Flieder, D., Koumbi, D., Testa, J., Eisenberg, B., von Mehren, M., and Godwin, A.K. Insulin-like growth factor 1 receptor: a potential therapeutic target for gastrointestinal stromal tumors. Proc. Natl. Acad. Science, USA 105(24):8387-8392, 2008. (┼Authors have equal contribution) PubMed
The Rink Laboratory at the Fox Chase Cancer Center is hiring an ambitious and self-motivated Postdoctoral Fellow to understand the mechanisms of resistance to small molecule inhibitors in Gastrointestinal Stromal Tumors (GIST) through both basic and translational studies. The goal of this laboratory is to translate these findings into novel treatments for GIST. The laboratory uses state-of-the-art technologies and collaborations with leading scientists and clinicians. Some examples of publications addressing these areas of interest are: Ye et al. (2021) JCI Insight; Zook et al. (2017) Clinical Cancer Research; Von Mehren et al. (2020) Clinical Cancer Research; Kozinova et al. (2021) Cancers. Additional details regarding the laboratory research can be found at https://www.foxchase.org/lori-rink.
As one of the four original cancer centers to receive comprehensive designation from the National Cancer Institute, Fox Chase Cancer Center has been at the forefront of cancer research for almost 90 years. We are home to excellent research facilities, top clinicians and scientists, and outstanding patient care. Our singular focus on cancer, which couples discovery science with state of the art clinical care and population health, remains the foundation of our work.
The scientist training programs at Fox Chase Cancer Center provide professional development opportunities in four core areas identified as crucial for successful careers in science, research, and health care including communication, leadership, teaching, and mentorship. Upon joining the program, graduate students and postdocs develop individual development plans to help guide their growth. Training throughout the year is supplemented with free professional development opportunities, including a robust ‘How To’ series, writing courses, networking, mentorship, and teaching opportunities, a trainee-led seminar series, a trainee-led annual Research Conference, and more. Postdocs at Fox Chase Cancer Center are supported by the Temple University Postdoc Association and the Office of Academic Affairs at Fox Chase, and are compensated with competitive pay and benefits.
In addition to the robust training program, scientists at Fox Chase Cancer Center benefit from being part of the rich scientific and biotech environment in the Philadelphia region. Many of our former trainees are now employees (and contacts) at nearby institutions and companies, including The Wistar Institute, Merck, GSK, AACR, and numerous others.
Email a CV, cover letter that states research interest(s) and goals, and the name of at least three references to [email protected]. Qualified candidates will then be invited to complete a job application.
The following ratings and reviews are based on verified feedback collected from independently administered patient experience surveys. The ratings and comments submitted by patients reflect their own views and opinions. Patient identities are withheld to ensure confidentiality and privacy. Learn more about our Patient Experience Ratings.

Dr. Lori Rink in a lab


Margaret von Mehren, MD

Patient comments